Caribou Biosciences Reports CB-010 ANTLER Phase 1 Trial Progress
-- Long-term durability observed for CB-010 allogeneic cell therapy at dose level 1 in ANTLER trial for r/r B-NHL --
-- ANTLER trial enrolling patients at dose level 3 --
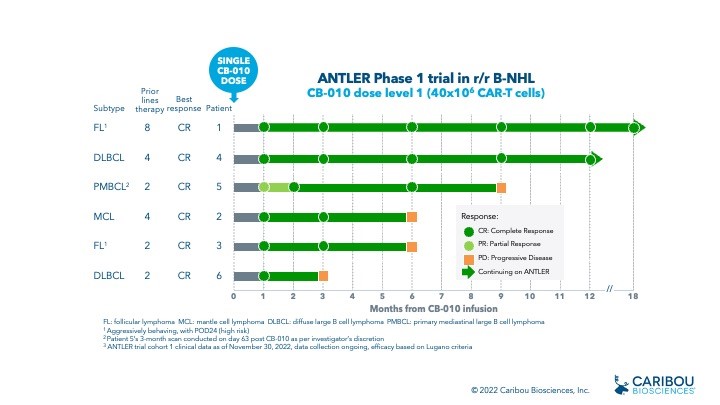
BERKELEY, Calif., Dec. 12, 2022 (GLOBE NEWSWIRE) -- Caribou Biosciences, Inc. (Nasdaq: CRBU), a leading clinical-stage CRISPR genome-editing biopharmaceutical company, today reported new 12-month clinical data from cohort 1 in the ongoing ANTLER Phase 1 trial, which show long-term durability following a single infusion of CB-010 at the initial dose level 1 (40x106 CAR-T cells). Cohort 1 results show:
- 6 of 6 patients achieved a complete response (CR) as best response
- 3 of 6 patients maintained a durable CR at 6 months
- 2 of 6 patients maintain a long-term CR at the 12 month scan and remain on the trial
- 18 months is the longest CR maintained to date in ANTLER, achieved by the first patient dosed with CB-010
- CB-010 was generally well tolerated with adverse events consistent with autologous or allogeneic anti-CD19 CAR-T cell therapies
Based on promising initial data, the U.S. Food and Drug Administration (FDA) granted CB-010 both Regenerative Medicine Advanced Therapy (RMAT) and Fast Track designations. In addition, Caribou has observed an encouraging safety profile for CB-010 at dose level 2 (80x106 CAR-T cells) with no dose-limiting toxicities (DLTs) in the 3 patients treated and is currently enrolling patients at dose level 3 (120x106 CAR-T cells). Caribou expects to provide an ANTLER trial update in 2023.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c8122817-d16a-4469-921f-2b0b7994b2d6
ANTLER Phase 1 trial of CB-010 at dose level 1
“With next-generation CRISPR genome-editing technology, the promise of allogeneic cell therapies has advanced significantly, and the early results seen in the ANTLER trial to date are a reflection of that potential,” said Rachel Haurwitz, Ph.D., Caribou’s president and chief executive officer. “The long-term durability at dose level 1 is comparable to autologous cell therapies and we believe CB-010 has the potential to set a new therapeutic bar for what allogeneic anti-CD19 CAR-T cell therapies can achieve. We are further encouraged by receiving RMAT and Fast Track designations for CB-010 from the FDA, which is a testament to both the encouraging initial ANTLER data and the need for novel therapies for patients with relapsed or refractory B-NHL.”
CB-010 is the first allogeneic anti-CD19 CAR-T cell therapy in the clinic with a PD-1 knockout, a genome-editing strategy designed to improve the persistence of antitumor activity by limiting premature CAR-T cell exhaustion.
“Patients with relapsed or refractory B cell non-Hodgkin lymphoma are in need of treatments that are immediately available and do not require burdensome or ineffective bridging therapies,” said Susan O’Brien, M.D., professor of medicine, Chao Family Comprehensive Cancer Center at University of California, Irvine, CA, and presenting investigator on the ANTLER clinical trial. “The early results from the ANTLER trial are promising and I look forward to enrolling additional patients in this trial to learn more about the potential of CB-010 as an off-the-shelf treatment option for patients with aggressive B-NHL who have a high unmet medical need.”
ANTLER is a Phase 1, open-label, multicenter clinical trial (NCT04637763) evaluating the safety and efficacy of the company’s lead allogeneic cell therapy, CB-010, in patients with r/r B-NHL. The trial includes Part A, a 3+3 dose escalation phase designed to evaluate safety of CB-010 at multiple dose levels and establish the recommended Phase 2 dose, and Part B, a dose expansion phase with the primary objective to determine tumor response after a single dose of CB-010. As permitted by the protocol, backfilling patients has begun at doses deemed well tolerated to increase the understanding of CB-010’s safety profile and antitumor activity and provide additional data for establishing a recommended Phase 2 dose.
ANTLER Trial-in-Progress Poster at ASH 2022
Today at the 64th Annual ASH meeting, a trial-in-progress poster is being presented to provide details of the design and objectives of the ANTLER Phase 1 trial for CB-010 in r/r B-NHL. Details of the poster presentation are as follows:
Title: A First-in-Human Phase 1, Multicenter, Open-Label Study of CB-010, a Next-Generation CRISPR-Edited Allogeneic Anti-CD19 CAR-T Cell Therapy with a PD-1 Knockout, in Patients with Relapsed/Refractory B Cell Non-Hodgkin Lymphoma (ANTLER Study)
Presenter: Susan O’Brien, M.D., professor of medicine, Chao Family Comprehensive Cancer Center at University of California, Irvine, CA
Session Name: 626. Aggressive Lymphomas: Prospective Therapeutic Trials: Poster III
Session Date: Monday, December 12, 2022
Presentation Time: 6:00 pm - 8:00 pm CST
Location: Ernest N. Morial Convention Center, Hall D
Abstract number: 4257
The poster presentation will be available for registered attendees on the ASH website and on Caribou’s website under Scientific Publications on Monday, December 12, 2022 at 9:00 am CST.
About CB-010
CB-010 is the lead product candidate from Caribou’s allogeneic CAR-T cell therapy platform and is being evaluated in patients with relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL) in the ongoing ANTLER Phase 1 trial. CB-010 is an allogeneic anti-CD19 CAR-T cell therapy engineered using Cas9 CRISPR hybrid RNA-DNA (chRDNA) technology to insert a CD19-specific CAR into the TRAC gene and knock out PD-1 to boost the persistence of antitumor activity. CB-010 is the first allogeneic CAR-T cell therapy in the clinic with a PD-1 knock out. CB-010 has been granted Regenerative Medicine Advanced Therapy (RMAT), Fast Track, and Orphan Drug designations. Additional information on the ANTLER trial can be found at www.clinicaltrials.gov using identifier NCT04637763.
About Caribou’s Novel Next-Generation CRISPR Platform
CRISPR genome editing uses easily designed, modular biological tools to make DNA changes in living cells. There are two basic components of Class 2 CRISPR systems: the nuclease protein that cuts DNA and the RNA molecule(s) that guide the nuclease to generate a site-specific, double-stranded break, leading to an edit at the targeted genomic site. CRISPR systems are capable of editing unintended genomic sites, known as off-target editing, which may lead to harmful effects on cellular function and phenotype. In response to this challenge, Caribou has developed CRISPR hybrid RNA-DNA guides (chRDNAs; pronounced “chardonnays”) that direct substantially more precise genome editing compared to all-RNA guides. Caribou is deploying the power of its Cas12a chRDNA technology to carry out high efficiency multiple edits, including multiplex gene insertions, to develop CRISPR-edited therapies.
About Caribou Biosciences, Inc.
Caribou Biosciences is a clinical-stage CRISPR genome-editing biopharmaceutical company dedicated to developing transformative therapies for patients with devastating diseases. The company’s genome-editing platform, including its proprietary Cas12a chRDNA technology, enables superior precision to develop cell therapies that are specifically engineered for enhanced persistence. Caribou is advancing a pipeline of off-the-shelf CAR-T and CAR-NK cell therapies for the treatment of patients with hematologic malignancies and solid tumors.
Follow us @CaribouBio and visit www.cariboubio.com.
“Caribou Biosciences” and the Caribou logo are registered trademarks of Caribou Biosciences, Inc.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements related to Caribou’s strategy, plans, and objectives, and expectations regarding its clinical and preclinical development programs, including its expectations relating to the timing of the release of additional patient data from its ANTLER Phase 1 clinical trial for CB-010. Management believes that these forward-looking statements are reasonable as and when made. However, such forward-looking statements are subject to risks and uncertainties, and actual results may differ materially from any future results expressed or implied by the forward-looking statements. Risks and uncertainties include, without limitation, risks inherent in the development of cell therapy products; uncertainties related to the initiation, cost, timing, progress, and results of Caribou’s current and future research and development programs, preclinical studies, and clinical trials; and the risk that initial or interim clinical trial data will not ultimately be predictive of the safety and efficacy of Caribou’s product candidates or that clinical outcomes may differ as more patient data becomes available; as well as other risk factors described from time to time in Caribou’s filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2021 and subsequent filings. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. Except as required by law, Caribou undertakes no obligation to update publicly any forward-looking statements for any reason.
| Caribou Biosciences, Inc. Contacts: |
Investors Amy Figueroa, CFA |
| afigueroa@cariboubio.com Media Peggy Vorwald, Ph.D. pvorwald@cariboubio.com |
| Investors and Media: |
| Elizabeth Wolffe, Ph.D., and Sylvia Wheeler |
| Wheelhouse LSA |
| lwolffe@wheelhouselsa.com |
| swheeler@wheelhouselsa.com |


